Photo Credit: Wikipedia Commons (left); CC0 (right)

Research Assistant

Research Assistant
Even though the coronavirus (COVID-19) outbreak originated outside of the United States’ borders, it has come to dictate American life. In an effort to quell the domestic outbreak and “flatten the curve” of the projected number of Americans who will contract the virus, the White House and the Centers for Disease Control and Prevention (CDC) have taken a variety of emergency measures, ranging from providing practical public health advice to declaring a national emergency.
As a frontline defender against viral outbreaks in the United States, the CDC has been actively leading the U.S. public response to COVID-19. Along with releasing travel notices, it has also deployed personnel to strategic points around the country and led laboratory efforts in developing diagnostic tests for public use. Its slow – and many weeks late – rollout on this latter front has damaged its image of competence and efficiency.
The White House has been working closely with the CDC and Congress to project an all-of-government approach to addressing the COVID-19 crisis and, thereby, reassure the American public. President Trump’s optimistic tone through February and early-March, with regard to tidying through the outbreak, has given way to a more serious tone of acceptance of the longer-lasting damage that will be felt economically as well as to Americans’ public health.
The U.S. Congress itself has been active in passing a variety of bills to provide access to additional emergency funds and help mitigate the financial burdens of businesses and families – H.R. 6074, which opened up $8.3 billion in funds, being the most notable legislated piece of action so far. At this time, efforts are on in Congress to pass a gargantuan US$1.8 trillion fiscal package but disagreements have surfaced on the package’s contents among Democrats and Republicans.
The United States – and the world – is still in the midst of the coronavirus crisis. The worst is still ahead. Diagnostic testing has belatedly come up-to-speed. Vaccine research and development has made a quick start, but many arduous months of effort remains ahead. Measures to enforce quarantining of infected individuals has yet to be grappled with and peak infection rates – and inflection points – are yet unknown. Fear and uncertainty persist. The coming months will prove to be a test of American unity and trust in the government’s ability to protect the American people’s way of life.
In a matter of weeks, the novel coronavirus (formally known as COVID-19 or SARS-CoV-2) has expanded from a local respiratory illness in Wuhan, China to a global pandemic that has wrought unprecedented health and socio-economic consequences across the world. International transportation is on ice, supply chains are severely disrupted, and the U.S. stock market has suffered single-day trading losses unseen since the Black Monday crash of October 19, 1987.
The Trump Administration and the Centers for Disease Control and Prevention (CDC) under the U.S. Department of Health and Human Services (HHS) have resolved to “flatten the curve” of the projected number of Americans who will contract COVID-19 across a lengthier time-horizon (this will depend, of course, on the infection rate) as soon as possible. Of late, other federal government agencies have also been called to join the containment and response efforts, with U.S. Vice President Mike Pence announcing on March 19 that the Federal Emergency Management Agency (FEMA), in particular, would begin taking a leading role in the coordination of the federal government’s response. Insofar as the White House and the HHS/CDC, they have taken a distinct set of measures, respectively, to prevent, contain and overcome the threat posed by COVID-19. As of March 2020, they can be summarized as:
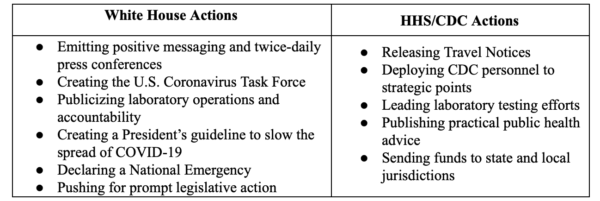
This Primer outlines the White House and the HHS/CDC actions taken in recent weeks to combat the COVID-19 pandemic within the United States. As developments regarding COVID-19 are still volatile, instead of providing a comprehensive list, this Primer identifies trends in actions taken by the White House and the CDC and their potential implications. Because the CDC was off-the-mark earlier in alerting the public to the potential spread of the epidemic, the Primer will lead off with the CDC’s alerts, actions and measures first.
As the foremost U.S. federal authority in regards to countering viral epidemics, the Centers for Disease Control and Prevention (CDC) is housed under the U.S. Department of Health and Human Services, and the agency has been at the helm in confronting and containing the potential outbreak of the coronavirus (COVID-19) on American soil. The intentions behind the CDC’s actions so far have followed the spirit of its mission statement:
“CDC works 24/7 to protect America from health, safety and security threats, both foreign and in the U.S…CDC fights disease and supports communities and citizens to do the same…To accomplish our mission, CDC conducts critical science and provides health information that protects our nation against expensive and dangerous health threats, and responds when these arise.”
The first public action that the CDC took to address the potential threat of the coronavirus–then unidentified–was to utilize its travel notice alerts system. On January 6, the CDC announced a Level 3 Warning Travel Notice for travel to China, recommending that travelers ‘Avoid Nonessential Travel.’ In an early nod to the virus’ potential threat level, the CDC did not announce a Level 1 Watch or Level 2 Alert travel notice before announcing a Level 3 Warning–the most elevated travel notice in the CDC’s rating system. A month later, the U.S. Department of State issued its own China Travel Advisory set at ‘Level 4: Do Not Travel.’
As COVID-19 spread outside of China, the CDC also set Level 3 Warnings concerning travel to Italy, South Korea, Malaysia, Iran, and cruise ship travels, a Level 2 Alert concerning Japan, and a Level 1 Watch concerning Hong Kong, often in conjunction with similar State Department advisories. High-risk travelers such as older adults and those with serious chronic medical conditions were given a Level 2 Travel Alert and advised to avoid any non-essential travel at the time. As March arrived and Europe became the epicenter of the COVID-19 outbreak, the CDC implemented Level 3 warnings for countries in the Schengen Area in Europe, which was then soon expanded to include the United Kingdom and Ireland.
The CDC also deployed officers and specialists to strategic locations, such as U.S. ports of entry (i.e. airports), CDC quarantine stations (i.e. U.S. military bases), and state and local health departments and hospitals deemed to be operating in higher-risk locations. CDC deployments were made in collaboration with the U.S. Department of Homeland Security (DHS), the World Health Organization (WHO), and state, local and national health authorities.
The first deployment occurred on January 20 and by February 24, 2020, the CDC had already reported a total of 1,336 of its staff members being involved in the COVID-19 response, 37% of whom having been deployed to the front-lines at 39 domestic and international locations. For example, on January 20 the CDC sent over 100 of its officials to 11 U.S. international airports to conduct screenings alongside the DHS. Flights from China were directed to land at one of these 11 airports so that passengers returning to the U.S. could go through a health screening before passing through customs.
As the number of confirmed viral cases jumped in China and began to spread across the world, the demand for diagnostic tests skyrocketed. Between January 18–when CDC laboratory testing began in earnest–and February 23, CDC laboratories used a “real-time reverse transcription–polymerase chain reaction (RT-PCR) to test 2,620 specimens from 1,007 persons for SARS-CoV-2.” By March 18, a total of 37,824 specimens were tested for SARS-CoV-2 by CDC labs (4,484) and other U.S. public health laboratories (33,340).
However, during the first few days of March, the first mass-produced diagnostic tests for COVID-19 developed in the Atlanta CDC laboratory were found to be “botched,” leading to widespread criticism of the CDC’s reliability and the government’s response. The late onset of mass-available diagnostic testing has been a critical failure on CDC’s part, given in particular COVID-19’s aggressive community transmission rate.
Alongside other measures that it conducts, the CDC also continuously published informational reports and recommendations directly related to containing the spread of COVID-19. The most popular and repeated guidance was to “thoroughly wash your hands” and “enact social distancing.” Both the CDC and HHS have also modified their websites so visitors can quickly and easily locate information on the epidemic.
For example, as part of its user-friendly format, the CDC offers concise information guides designed for the general public and health professionals. Topics include: hospital and healthcare facility preparedness, healthcare infection prevention and control, precautions on preparing communities for potential spread of COVID-19, evaluating and reporting persons under investigation (PUI), nonpharmaceutical interventions for community preparedness and outbreak response, optimizing the supply of N95 respirators, and actions for active monitoring of potentially exposed persons.
Beginning March 4, the Department of Health and Human Services (HHS) announced that it would be awarding funds to states and local jurisdictions to aid the domestic response to contain and monitor the spread of COVID-19. US$25 million was to be awarded to help provide assistance to states and local jurisdictions that were in immediate need of resources to monitor travelers, required laboratory and medical supplies as well as resources for staffing and infection control. Another US$10 million was to be awarded to state and local jurisdictions to build on existing influenza activities and surveillance systems.
This initial funding was provided to the CDC through the HHS Secretary’s Transfer. With more additional funding in the pipeline, more states and local jurisdictions will be in line to receive support from the CDC and HHS.
On February 25, Alex Azar, Secretary of the U.S. Department of Health & Human Services, held a briefing providing updates on the COVID-19 outbreak risks for the American public as well as an update on actions that the Trump Administration has taken in response to the global outbreak. Azar stated that the Trump administration’s aggressive and transparent early response to the outbreak had bought the U.S. valuable time in order to monitor and prepare for a possible outbreak within the U.S. borders. That valuable time though has been frittered away by the grave shortfalls on the testing and diagnostic front – in turn, leading to a rocketing number of community-based transmissions which is just now being detected.
President Donald Trump and his administration have sought to tackle the coronavirus outbreak in the U.S. by utilizing various methods, including long-drawn daily press conferences (to provide transparency and manage public messaging), proclaiming travel bans, and most importantly, by creating the U.S. Coronavirus Task Force.
Until early-to-mid March, almost every public address by the White House on the coronavirus outbreak was fueled by optimism and assurances that the government had firm control over the situation. On January 22, President Trump said that “we have it totally under control….it’s going to be just fine.” In early February, President Trump announced that the U.S. has taken actions to “shut down” the coronavirus threat and on February 25 he stated that “we have contained this…we have done a good job in the United States.”
As cases nevertheless began to sprout up in the U.S., President Trump, Vice President Pence, and members of the Coronavirus Task Force held a press conference on February 27 assuring that the administration is “ready to adapt” and is ready to take any necessary actions if the disease spreads. President Trump stated that there is “good bipartisan spirit” regarding the negotiations on funding for the COVID-19 response. A day later, President Trump said “it’s going to disappear. One day, it’s like a miracle, it will disappear.”
Positive messaging thereafter focused on vaccine development and diagnostic testing. On March 3, President Trump visited the Vaccine Research Center at the National Institutes of Health (NIH) to support the ‘front line’ experts who are trying to develop a vaccine for the coronavirus. In his remarks, he thanked doctors and scientists at the NIH for doing a “fantastic job.”
At the signing of H.R. 6074, the “Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020” on March 6, President Trump stated that the $8.3 billion bill would support the virus outbreak containment and response efforts. He also noted that Integrated DNA Technologies (IDT), a private contractor, was currently working with the CDC for the production of 2019-novel coronavirus test kits and which would soon show results. Later that day, President Trump visited the CDC lab in person, where he held an impromptu news conference that lasted 47 minutes to address concerns. “It will end. People have to remain calm,” President Trump said while touring the facility. He also assured the people that “anybody that needs a test, gets a test. They’re there.”
Earlier, on March 1st, as part of the Administration’s efforts to contain the spread of COVID-19, President Trump had tweeted that passengers from certain countries that were being screened ‘prior to boarding’ would also now be screened when they arrive in the U.S. The president’s aim broadly had been to reassure the general public that the U.S. was taking aggressive actions to prevent the spread of the virus from other nations.
Continuing through early-and-mid March, President Trump repeated – not entirely correctly as time has shown – that “we have tremendous control over” the coronavirus spread and asked Americans not to hoard essential supplies but to “just stay calm. It will go away.” However, the media noted a qualitative shift in the president’s tone beginning March 16 when he announced a new “15 Days to Slow the Spread” Guideline, which includes a schooling and working from home recommendation and avoiding gatherings of more than 10 people. On March 17, Trump stated that “I’ve always known this is a real–this is a pandemic” and urged his countrymen and women to adopt a more serious approach to the situation. “We’re all in this together. It’s something that nobody expected,” he said. Furthermore, three days earlier, he declared March 15, 2020, a National Day of Prayer for all Americans Affected by the Coronavirus.
On January 29, the White House announced that President Trump had formed the U.S. Coronavirus Task Force. Secretary of Health and Human Services Alex Azar was to lead this task force along with others senior members of the Administration, such as the Assistant to the President for National Security Affairs, Robert O’Brien, Director of the CDC, Dr. Robert Redfield, and Dr. Anthony Fauci, the Director of the National Institute of Allergy and Infectious Diseases (NIAID), housed within NIH. As described by the White House, “[t]he Task Force will lead the Administration’s efforts to monitor, contain, and mitigate the spread of the virus, while ensuring that the American people have the most accurate and up-to-date health and travel information.”
Almost a month later, on February 26, President Trump appointed Vice President Mike Pence to lead the government’s COVID-19 task force. As cases of COVID-19 have risen in the U.S. and around the globe through February and into March, more experts, such as the U.S. State Department’s global director on AIDS, Dr. Deborah L. Birx, have been added to the Task Force.
Like the CDC, the White House also addressed travel in the face of the epidemic’s outbreak. When the number of confirmed cases was rising in China, on January 31, President Trump first issued Proclamation 9984 which implemented a travel ban preventing individuals who have visited China in the previous 14 days from entering the U.S. The proclamation went into effect on February 2 at 5 pm EST. Almost a month later on February 29, he issued Proclamation 9992 which expanded this restriction to the Islamic Republic of Iran.
On March 11, Trump made an address to the nation regarding the COVID-19 global outbreak. In his address, he issued Proclamation 9993, banning all travel for the next 30 days from the Schengen Area of Europe–consisting of 26 European nations–which went into effect on March 13 at midnight. The following day, on March 14, he expanded this ban to include travelers who had visited the United Kingdom and Ireland within 14 days of trying to enter the U.S.
President Trump has also commented on state and local actions related to travel, such as the first ‘containment area’ in the U.S. On March 10, New York governor Andrew Cuomo declared a one-mile radius “Containment Area” in New Rochelle/Westchester, New York, due to a “cluster” of 108 cases. Schools, houses of worship, and other places with large gatherings are to be closed from March 12 to March 25. Trump said on March 12, the day the National Guard arrived in New Rochelle, that “they’re doing the right thing.”
The Trump administration has shown support for scientific efforts to combat COVID-19 as well as ensure that laboratories remain accountable for their operations. It recently ordered an “independent investigation” of the CDC headquarters and laboratory in Atlanta, Georgia where the COVID-19 test kits were made and found to be wanting in early-March due to a ‘manufacturing issue’ and potential contamination.
The following day, the White House held another press briefing led by U.S. Food and Drug Administration (FDA) Commissioner Stephen M. Hahn. His speech was dedicated to “being transparent” and providing the “most comprehensive and up to date information about the status of diagnostic tests,” including numerical data and expansive descriptions of the FDA’s methodology. He concluded by assuring the public that the FDA was “dedicating all available resources” and taking regulatory steps to “encourage the development of new diagnostic tests.”
On the afternoon of March 13, President Trump held an almost two-hour-long press conference in the White House Rose Garden to provide updates of the Administration’s countermeasures against the coronavirus pandemic. In the course of the press conference, he announced his consequential decision to officially declare a National Emergency, which opens up access to additional resources and choices. Following this declaration, the President exhorted his Coronavirus Task Force leadership as well as the CEOs of private corporations (including Walgreens, CVS, Walmart, Target, and LapCorps) to jointly cooperate and bring a swift end to the spread of the pandemic in the United States. One key area of emphasis was on developing and distributing diagnostic tests to “drive-by” stations by the following week for public availability and use.
“Ten days ago, I brought together the CEOs of commercial labs at the White House and directed them to immediately begin working on a solution to dramatically increase the availability of tests….As a result of that action, today we’re announcing a new partnership with private sector to vastly increase and accelerate our capacity to test for the coronavirus. We want to make sure that those who need a test can get a test very safely, quickly, and conveniently.”
- President Donald Trump
As confirmed COVID-19 cases in the U.S. have risen, the White House has audibly pushed Congress to enact legislation to, both, fund measures to combat the spread of the virus and also cushion the economic blow of the widely-anticipated recession that is expected to follow. For instance, on March 9, President Trump announced that he will be asking Congress to pass legislation for a possible payroll tax cut and relief for hourly workers. At this time of writing, the Administration is in advanced discussions with lawmakers to enact a trillion-dollar-plus economic stimulus package.
Until the weekend of March 22-23rd, the U.S. Congress had displayed admirable unity in crafting bipartisan legislation to cushion the economic blow of COVID-19. Markets have plummeted since the global outbreak of COVID-19, forcing Congress to debate legislative responses that boost the economy or alleviate the impact on the hardest-hit industries and individuals. To date, the largest of these actions passed by Congress has been H.R. 6074 “Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020,” signed into law on March 6 by President Trump (see box). Among other acts with bipartisan support, President Trump signed H.R. 6201, the “Families First Coronavirus Response Act” into law on March 18. This bill provides paid sick leave and free coronavirus testing, expands food assistance and unemployment benefits, and requires employers to provide additional protections for health care workers.
Note: Update and addition on March 25: “Of late, other federal government agencies have also been called to join the containment and response efforts, with U.S. Vice President Mike Pence announcing on March 19 that the Federal Emergency Management Agency (FEMA), in particular, would begin taking a leading role in the coordination of the federal government’s response.”
After passing through the 116th U.S. Congress, on March 6 President Trump signed into law H.R. 6074, also known as the “Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020.” The proposed funding granted by this Act increased to $8.3 billion from the original $2.5 billion proposed by the Trump Administration. This act provides additional fiscal year 2020 emergency supplemental funding “for necessary expenses to prevent, prepare for, and respond to coronavirus” at the local, state, national and international levels.
The provisions therein stipulate funding for stockpiles, salaries, vaccine development, therapies, economic disaster loans, U.S. manufacturing of platform-based technologies, front-line training, and telehealth programs to support remote doctor consultations.
The Act, which classifies coronavirus as a “disaster” [Title II], also includes accountability by demanding various “detailed spend plan[s] of anticipated uses of funds.” For example, the HHS must submit a plan to Congress within 30 days [General Provisions Sec. 305.] of the enactment of this Act and provide a report to the Committees on Appropriations of the House and Representatives and the Senate every 14 days [Title III]. All provisions are to remain available until either September 30, 2022, or September 30, 2024.
At this time of writing, the earlier bipartisanship on display has shown signs of fraying with disagreements evident among Democrats and Republicans as they attempt to craft a gargantuan fiscal stimulus package of the order of US$1.8 trillion. The key planks of the bill, S. 3548, are: (a) loans to small businesses, (b) emergency loans to distressed large businesses, and (c) direct payment to adult U.S. citizens and residents below a certain income threshold. The bill’s key sticking points are the US$500 billion in corporate aid allocation which lacks basic transparency and related safeguards and the bill’s excessive tilt in favor of U.S. airline companies – they are due to receive almost two-thirds of what the U.S. hospital sector is due to receive by way of loan programs.
The CDC and the White House have taken numerous aggressive actions, especially of late, in order to slow, contain, and mitigate the spread of COVID-19. Restricting and banning travel bought the U.S. some amount of time to prepare for a domestic outbreak. As cases were confirmed in the United States, the CDC deployed personnel and published information guides designed to inform public health professionals and the general public on how to protect oneself as well as others. The White House addressed the nation with positive messaging to assure Americans that this virus was under control but has now, facing reality, shifted to a more somber tone. This led to President Trump’s mid-March landmark declaration of a National Emergency. Medical experts and scientists have struck up private-public partnerships in order to lead testing efforts as well as develop a vaccine, although it is projected that it may take up to 12-18 months for the vaccine to be available to the public. Congress, too, has gotten into the act, directing $8.3 billion towards combatting the pandemic and supporting the troubled economy, and much, much more by way of Congressional funding is expected to follow.
This situation remains tense nonetheless – in no small part, due to the failure to roll out early diagnostic testing on an industrial scale as well as the complacently self-congratulatory tone adopted by the White House through February and early-March. At this time, measures to track and enforce mass quarantines of sick and infected members of the public has not even been fully grappled with. Nevertheless, the CDC and the Coronavirus Task Force continue to provide frequent status updates and have (finally) succeeded in alerting the American public to the domestic public health threat, given the fact that there are still many unknowns. And the White House remains resolutely focused on “flattening the curve” of the domestic coronavirus outbreak and buoying the public’s spirit through this difficult time.

The Institute for China-America Studies is an independent nonprofit, nonpartisan research organization dedicated to strengthening the understanding of U.S.-China relations through expert analysis and practical policy solutions.
1919 M St. NW Suite 310,
Washington, DC 20036
icas@chinaus-icas.org
(202) 968-0595
© 2026 INSTITUTE FOR CHINA-AMERICA STUDIES. ALL RIGHTS RESERVED.
U.S. “Big Beautiful Bill” Redraws Map for Chinese Exporters